
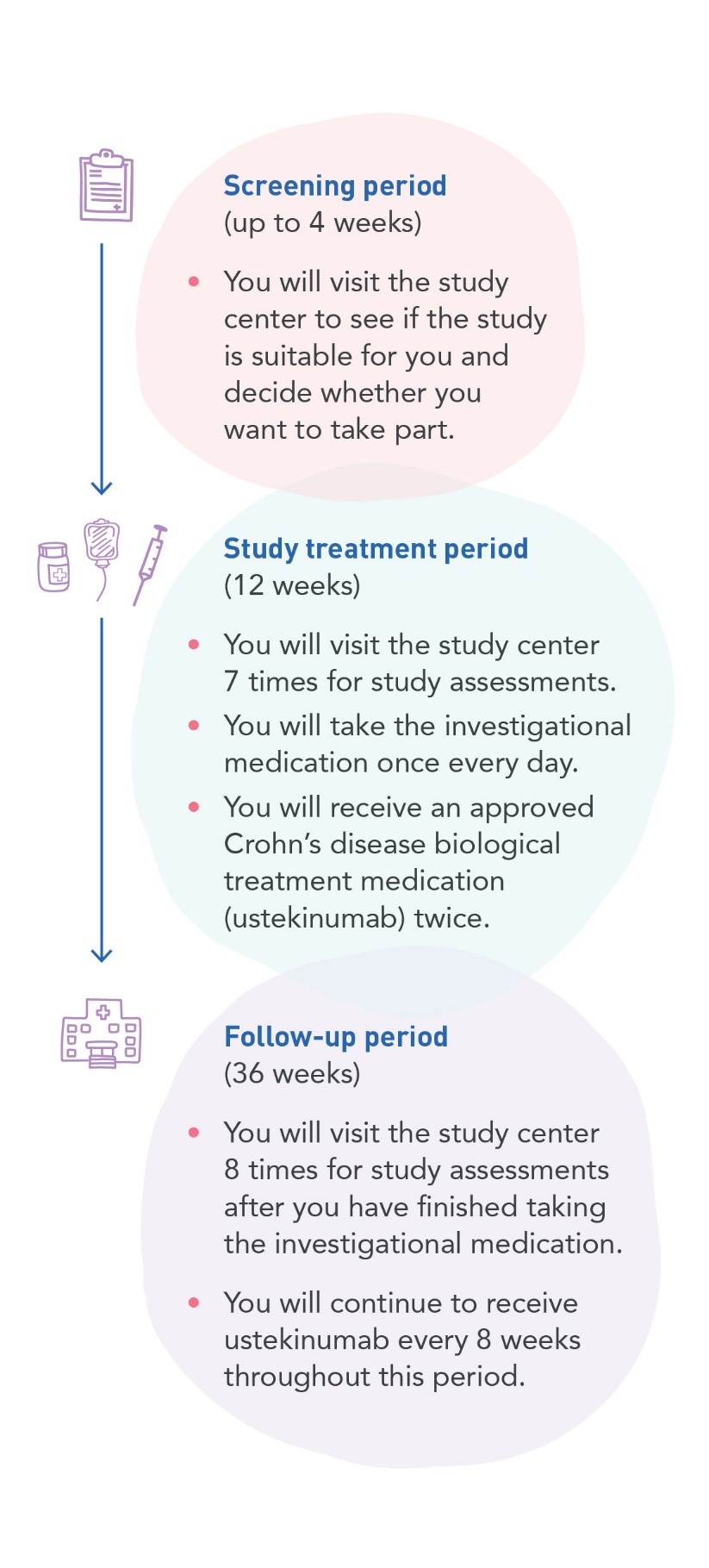
The InChargeStudy is a clinical research study that will include up to 76 people with Crohn’s disease (CD). The study will look at how safe an investigational medication is and whether it works when taken with a CD medication (ustekinumab) that is already approved for use.
The study is sponsored by Boehringer Ingelheim. You can find participating investigative sites at this website: www.ClinicalTrials.gov using search term 1425-0003.
Crohn’s - who’s in charge?
If your Crohn’s disease is out of control, join a clinical study aiming to put you back in charge!
About the InCharge
The InChargeStudy is a clinical research study that will include up to 76 people with Crohn’s disease (CD). The study will look at how safe an investigational medication is and whether it works when taken with a CD medication (ustekinumab) that is already approved for use. The study is sponsored by Boehringer Ingelheim. You can find participating investigative sites at this website: ClinicalTrials.gov using search term 1425-0003.b
Why is this study important?
CD is a condition that causes inflammation in the digestive tract. There are multiple treatments available for people with CD. However, for many people with CD, these treatments do not work, only partly work, or stop working over time. Therefore, it is important to research new treatment options that work differently from the treatments that are currently available. The investigational medication is a medication taken by mouth, which is a kinase inhibitor which is intended to blunt the inflammatory response to microbiome in the gut. It is hoped that blocking this protein will reduce inflammation in the intestines and lessen the symptoms of CD.
Who can take part?
You may be able to take part if you:
• are 18–75 years of age
• have moderate to severe CD
• have taken an anti-tumour necrosis factor antibody medication for CD that did not work well enough, stopped working, or had to be stopped because of side effects or for other reasons.
Disclaimer:
This trial-related material is approved by ethics committees and is officially published for the following countries: Belgium, Czech Republic, Denmark, Germany, Italy , Poland and Spain.
The trial itself is approved and recruiting in the following countries: Belgium, Czech Republic, Denmark, Germany, Hungary, Italy, Netherlands, Poland, Spain and USA.